High-voltage anode-free sodium–sulfur batteries
TL;DR
Researchers developed a high-voltage (3.6 V) anode-free sodium-sulfur battery using a novel S/SCl4 cathode chemistry and sodium dicyanamide electrolyte. This design achieves high energy densities up to 2,021 Wh/kg and addresses previous limitations of low discharge voltages and excess sodium metal requirements.
Key Takeaways
- •A 3.6 V class anode-free Na-S battery was created using S/SCl4 cathode chemistry and NaDCA electrolyte
- •The design enables high energy densities (up to 2,021 Wh/kg) and power densities (23,773 W/kg)
- •Incorporating a Bi-COF catalyst improves S/SCl4 conversion, achieving 1,206 mAh/g discharge capacity
- •The battery shows promise for grid storage and wearable electronics with estimated cost of $5.03/kWh
- •Addresses key limitations of traditional Na-S batteries: low discharge voltages and excess sodium metal requirements
Tags
Abstract
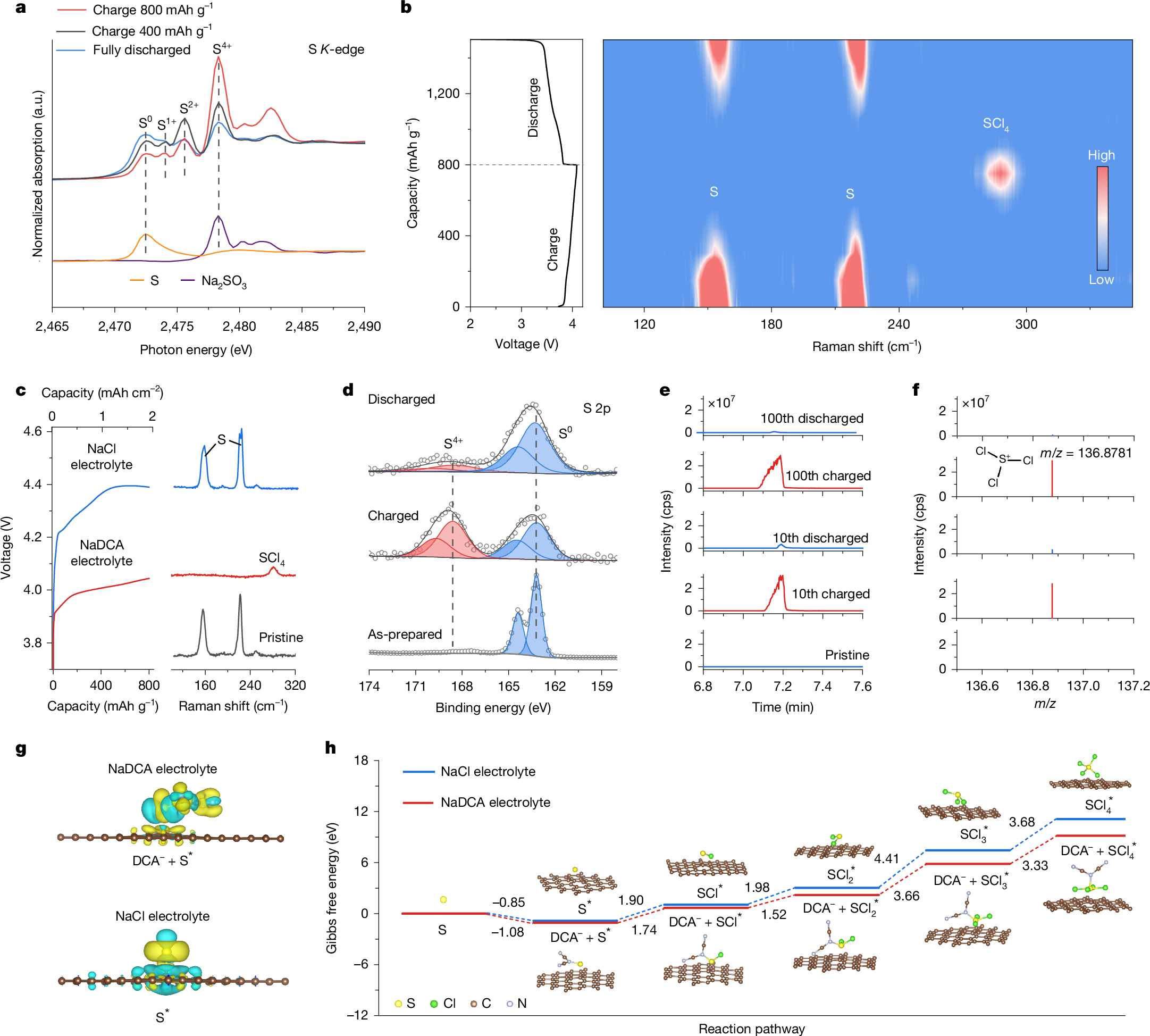
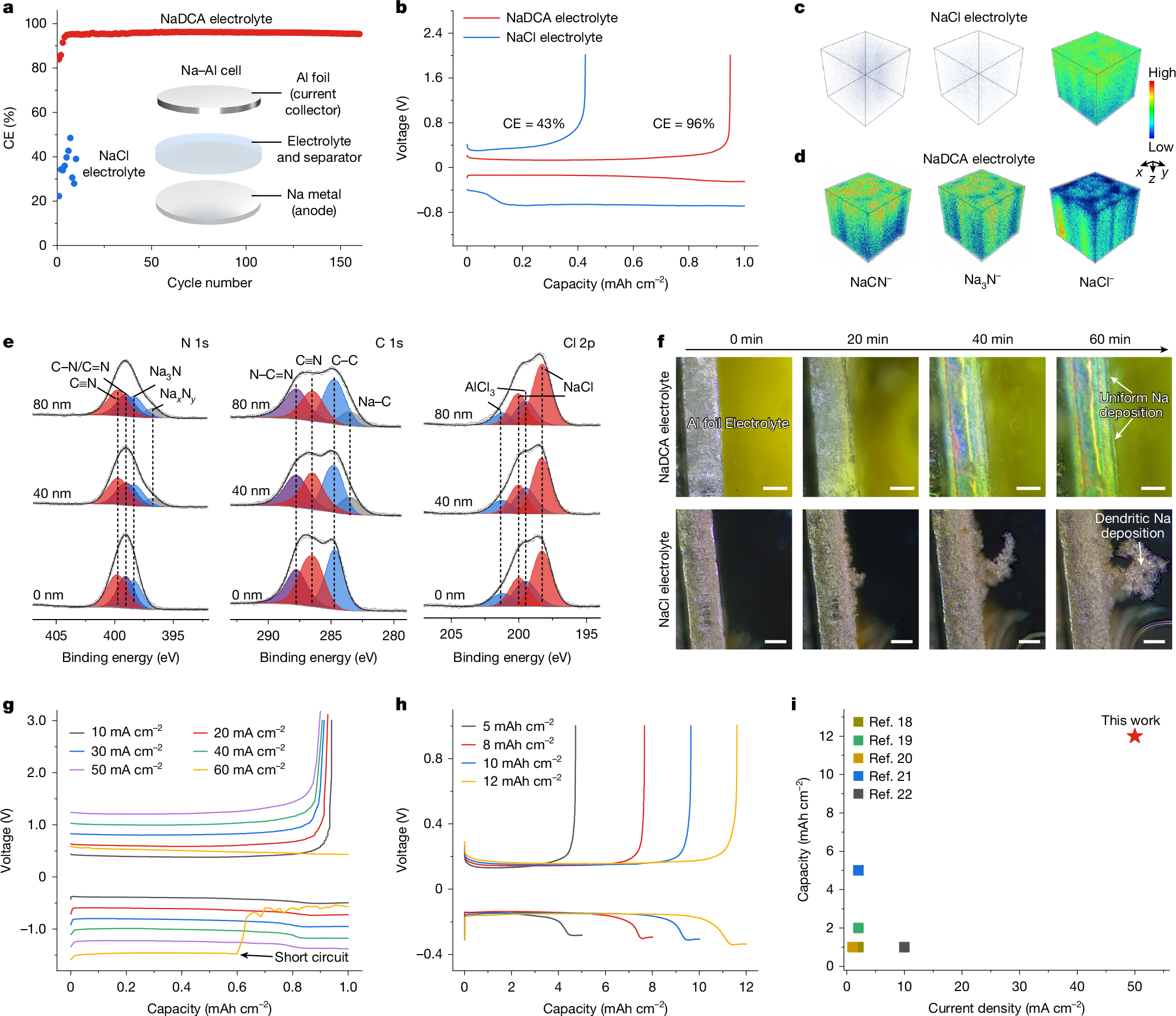
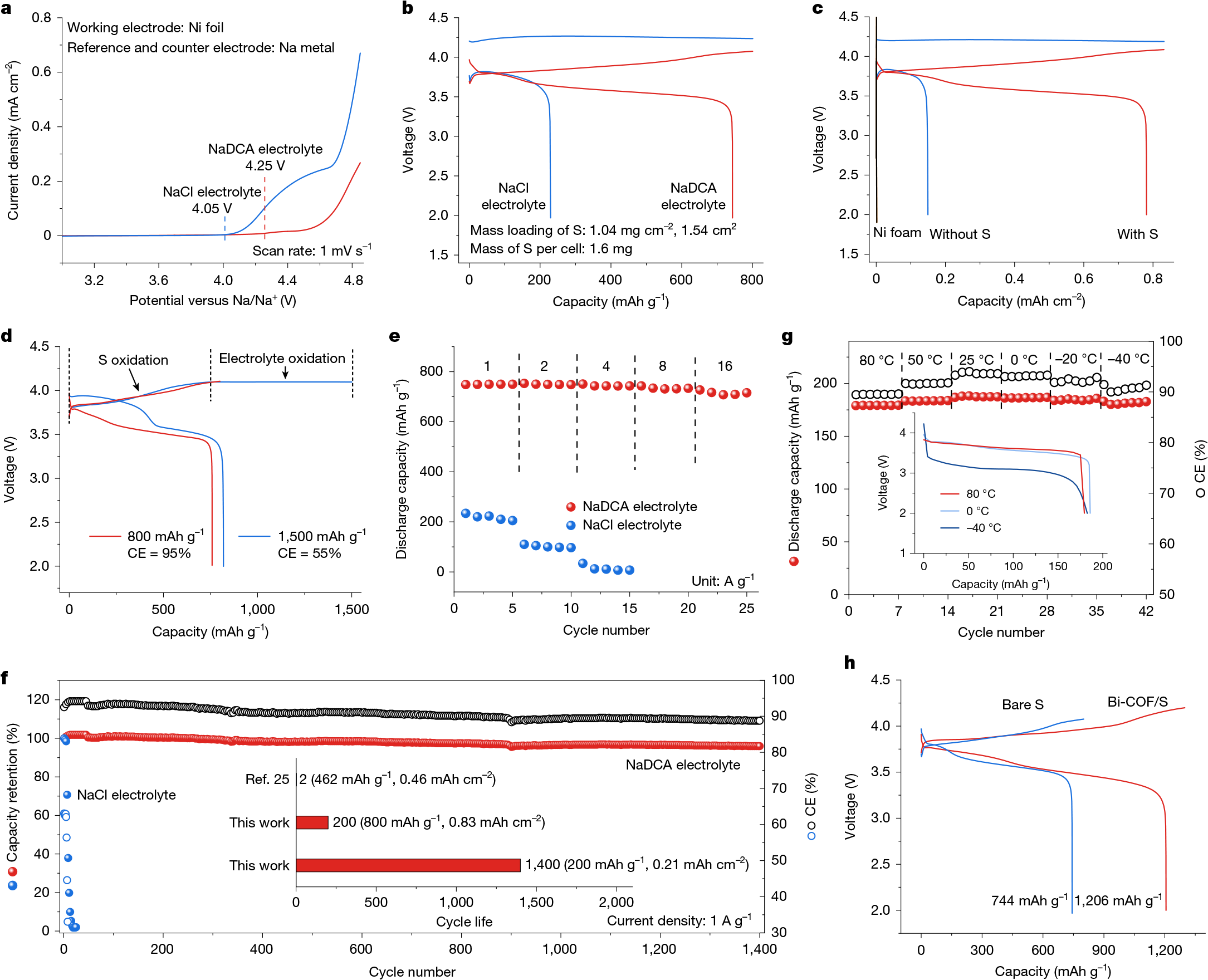
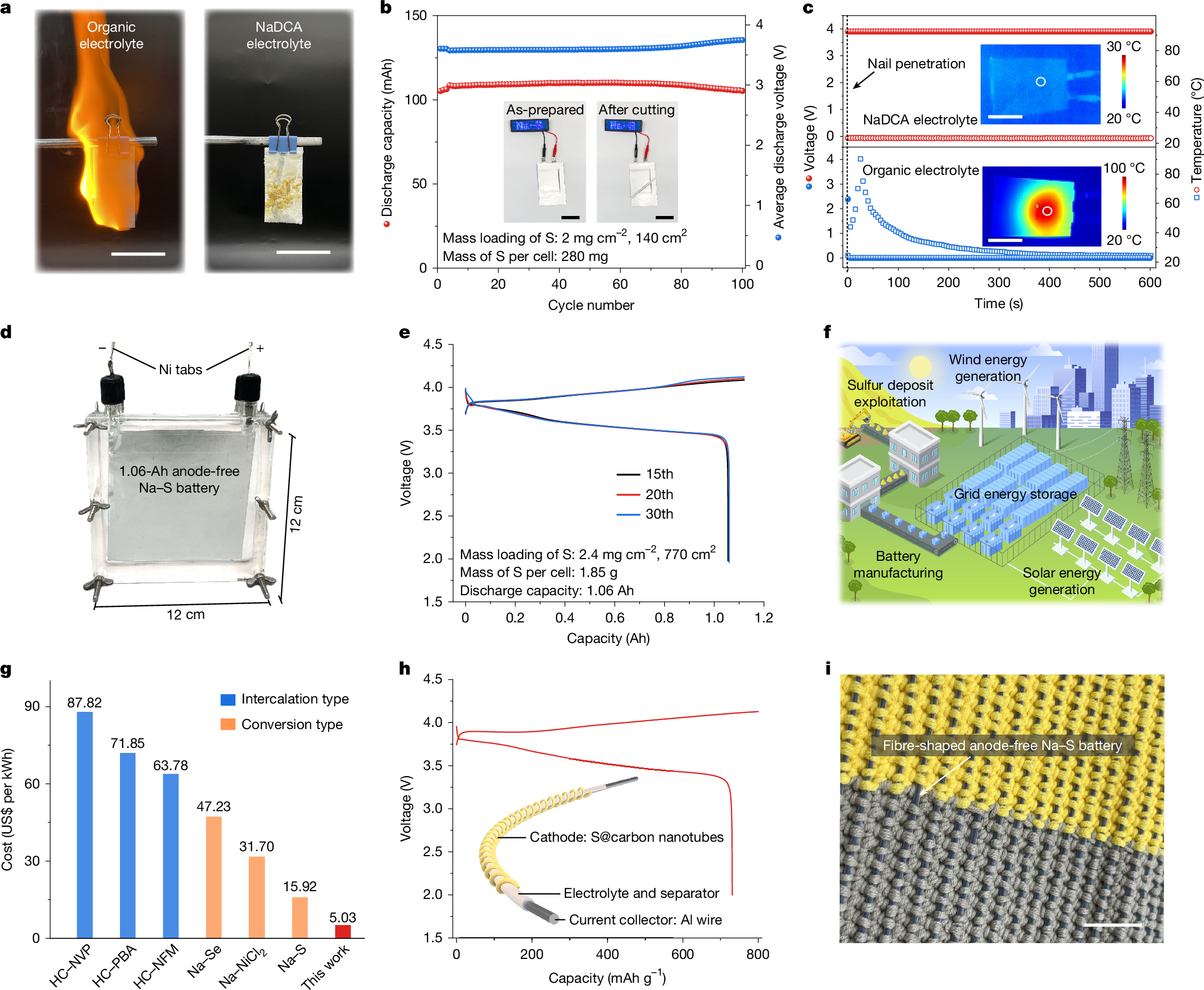
Room-temperature sodium–sulfur (Na–S) batteries offer a sustainable energy storage solution to conventional lithium (Li)-based systems1,2,3, owing to the high element abundances and theoretical electrochemical performance4,5. However, their practical applications have been severely hindered by the low discharge voltages and the need for largely excessive Na metal anode6,7,8. Here we report a 3.6 V class Na–S battery featuring a high-valence sulfur/sulfur tetrachloride (S/SCl4) cathode chemistry and anode-free configuration. We show that sodium dicyanamide (NaDCA) can simultaneously unlock reversible S/SCl4 conversion and Na plating/stripping in a non-flammable chloroaluminate electrolyte. This design enables the maximum energy and power densities of 1,198 Wh kg−1 and 23,773 W kg−1, respectively, calculated on the basis of the total electrode mass including both the cathode and the anode. Also, we demonstrate facilitated S/SCl4 conversion by incorporating a bismuth-coordinated covalent organic framework (Bi-COF) catalyst (8 wt% loading) into the S cathode, which realizes an impressive discharge capacity of 1,206 mAh g(sulfur+catalyst)−1, contributing to a maximum energy density of 2,021 Wh kg−1 calculated on the basis of the total electrode mass. With an estimated cost of US$5.03 per kWh and excellent scalability, our anode-free Na–S battery shows promise in grid energy storage and wearable electronics.
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Intercalation-type catalyst for non-aqueous room temperature sodium-sulfur batteries
Directing selective solvent presentations at electrochemical interfaces to enable initially anode-free sodium metal batteries
A Mo5N6 electrocatalyst for efficient Na2S electrodeposition in room-temperature sodium-sulfur batteries
Data availability
The data that support the findings of this study are available from the corresponding author on request.
References
Liu, R. et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature 626, 98–104 (2024).
Pan, H. et al. Non-encapsulation approach for high-performance Li–S batteries through controlled nucleation and growth. Nat. Energy 2, 813–820 (2017).
Liao, M. et al. Hybrid polymer network cathode-enabled soluble-polysulfide-free lithium–sulfur batteries. Nat. Sustain. 7, 1709–1718 (2024).
Bai, R. et al. Preferable single-atom catalysts enabled by natural language processing for high energy density Na-S batteries. Nat. Commun. 16, 5827 (2025).