Neuro-epithelial circuits promote sensory convergence and intestinal immunity
TL;DR
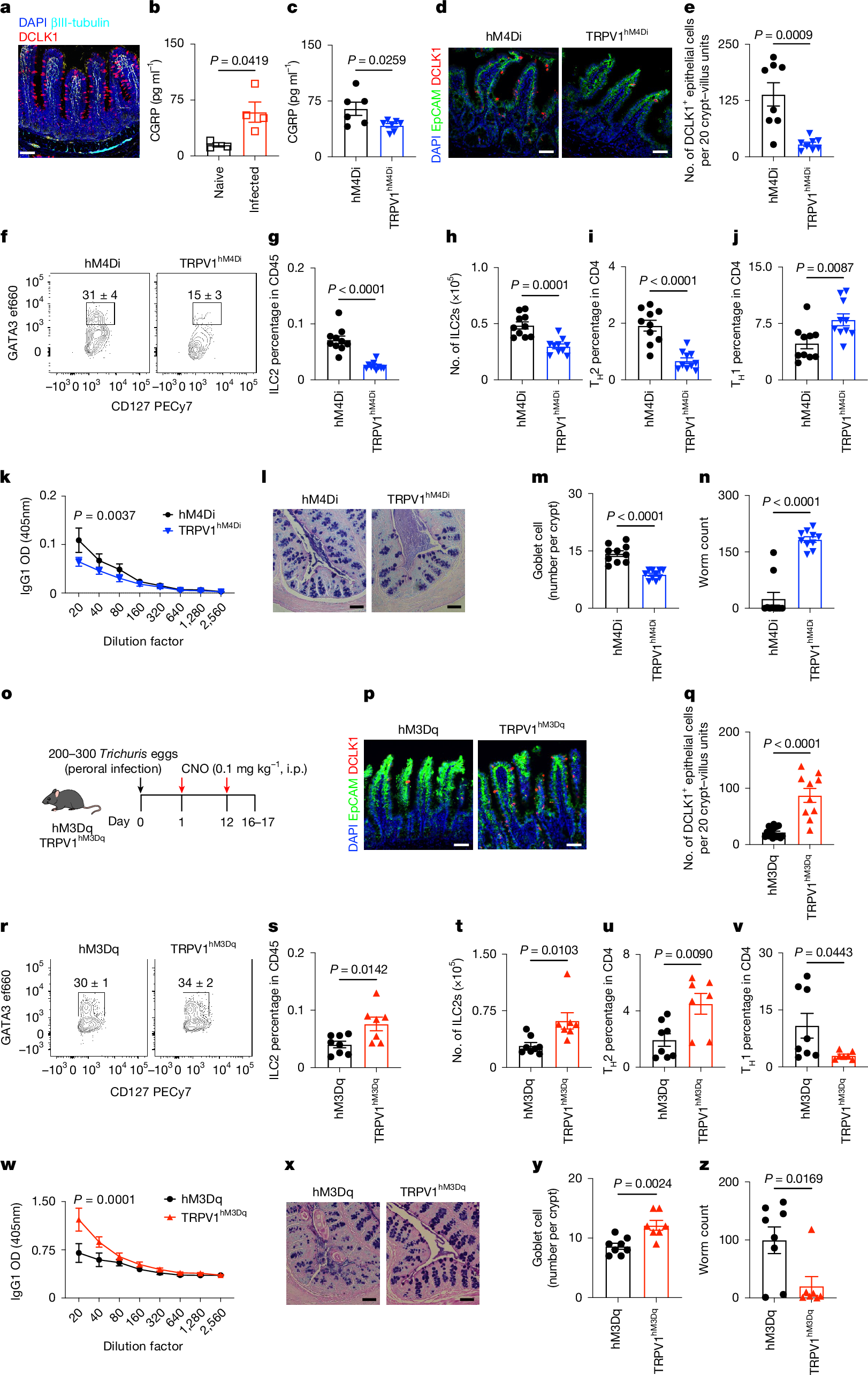
TRPV1+ pain-sensing nociceptors coordinate with intestinal tuft cells to drive type 2 inflammation and immunity against helminths. Activation of these neurons promotes epithelial cell proliferation and tuft cell accumulation via CGRP signaling. This neuro-epithelial circuit is crucial for sensory integration and protective immune responses.
Key Takeaways
- •TRPV1+ nociceptors initiate a cascade with chemosensory tuft cells to regulate type 2 inflammation and anti-helminth immunity.
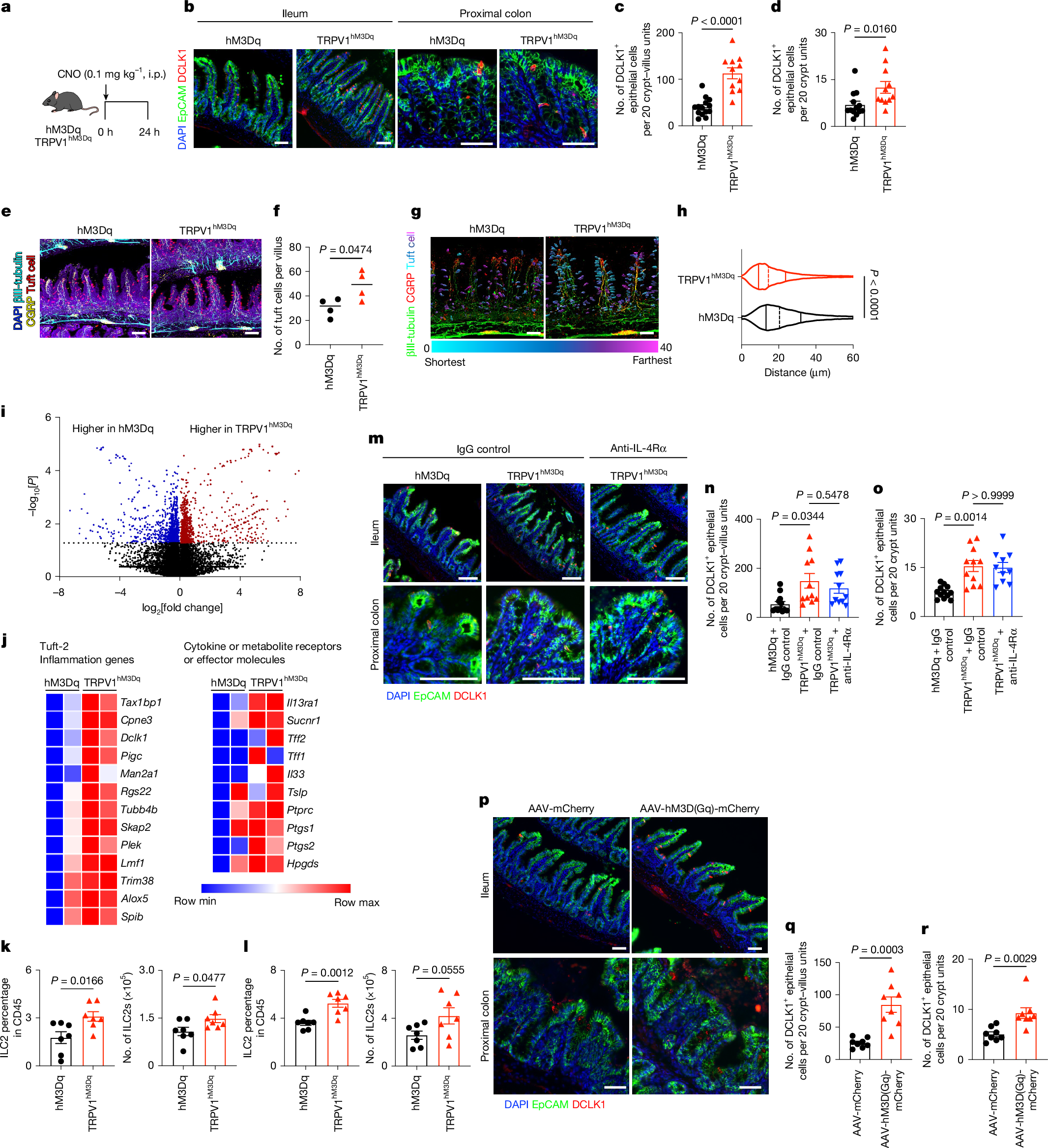
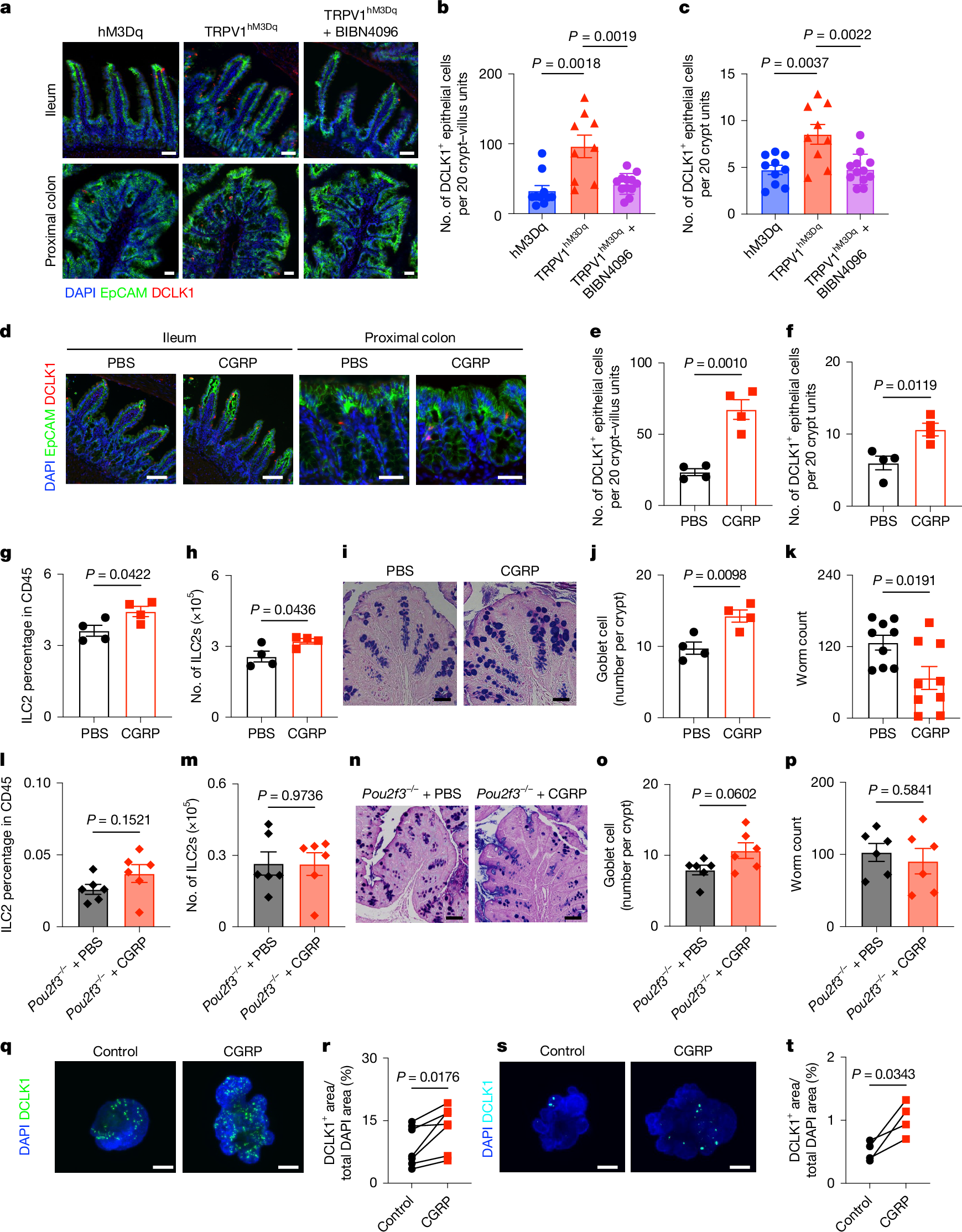
- •Chemogenetic activation of nociceptors increases CGRP expression, tuft cell accumulation, and protective immunity, while silencing reduces these effects.
- •CGRP receptor expression in intestinal epithelial and tuft cells is essential for tuft cell responses and type 2 immunity to helminth infection.
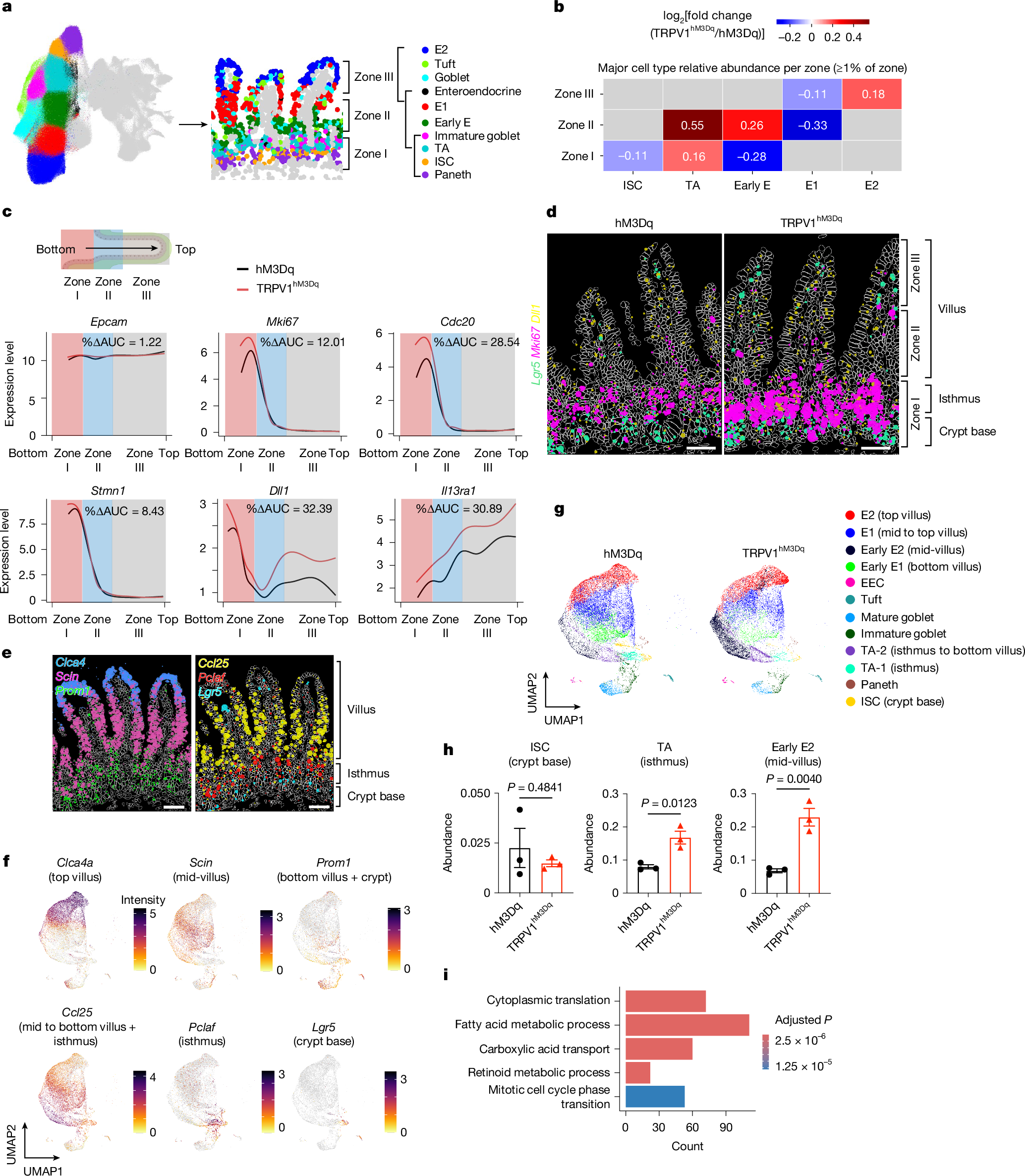
- •Spatial and single-cell RNA sequencing shows nociceptor activation drives rapid epithelial progenitor proliferation and differentiation.
- •The study identifies a neuronal-epithelial tuft cell circuit as a key upstream determinant of type 2 immunity and tissue adaptation.
Tags
Abstract
Type 2 inflammation at barrier surfaces is an evolutionarily conserved response that promotes immunity to helminth parasites, allergic inflammation and tissue repair1,2,3,4. Direct sensing of environmental triggers by epithelial cells initiates type 2 inflammation, and signals derived from neurons can modulate immune responses5,6,7,8. However, how diverse sensory inputs from epithelial, neuronal and immune cells are coordinated and integrated remains unclear. Here we identify that TRPV1+ pain-sensing nociceptors co-opt chemosensory epithelial tuft cells to initiate a cascade of tissue responses that drive type 2 inflammation. Chemogenetic silencing or chemical ablation of TRPV1+ nociceptors results in a significant reduction in intestinal tuft cells and defective anti-helminth type 2 immunity. By contrast, chemogenetic activation of TRPV1+ nociceptors leads to remodelling of CGRP+ nerve fibres, significantly increased CGRP expression, enhanced tuft cell accumulation and protective anti-helminth type 2 immunity. Using spatial transcriptomic and single-cell RNA sequencing analyses, we reveal that nociceptor activation promotes rapid epithelial progenitor cell proliferation and differentiation. Mechanistically, intestinal epithelial cell-intrinsic and tuft cell-intrinsic expression of CGRP receptor subunits are required for tuft cell responses and type 2 immunity to helminth infection. Together, these results identify sensory convergence of a neuronal–epithelial tuft cell circuit as a critical upstream determinant of type 2 immunity and tissue adaptation.
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Nociceptor neurons affect cancer immunosurveillance

Nociceptor-immune interactomes reveal insult-specific immune signatures of pain
Data availability
All data necessary to understand and evaluate the conclusions of this paper are provided in this Article or the Supplementary Information. The bulk RNA-seq and the scRNA-seq data are available at the European Nucleotide Archive under accession number PRJEB101609. The spatial transcriptomic data are available at the BioImage Archive under accession number S-BIAD2351. Other data are available from the corresponding authors upon appropriate and reasonable request. Source data are provided with this paper.
Code availability
Code used for sequencing and spatial transcriptomic analyses will be made available upon request.
References
Gieseck, R. L. 3rd, Wilson, M. S. & Wynn, T. A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76 (2018).
Hammad, H., Debeuf, N., Aegerter, H., Brown, A. S. & Lambrecht, B. N. Emerging paradigms in type 2 immunity. Annu. Rev. Immunol. 40, 443–467 (2022).
Locksley, R. M. Asthma and allergic inflammation. Cell 140, 777–783 (2010).